Atomic absorption method is one of the most accurate and easy methods for quantitative analysis of elements. In the Atomic Absorption method, minerals are first digested using different methods such as acidic, alkaline or aqueous. The choice of solvent depends on the element we want to analyze. For example, the suitable solvent for copper oxide mineral is sulfuric acid and the suitable solvent for gold containing mineral is sodium cyanide. After dissolving the desired element and ionizing it, they are exposed to light, which is usually produced by a hollow cathode lamp.
Here, free atoms and ions are excited by energy absorption (light absorption). A part of the light that is not absorbed by the material passes through the atoms and moves towards the detector. From here, the detector measures the intensity of the light beam and converts it into absorption data. Finally, by comparing the intensity of the emitted light and the intensity of the reflected light, it is determined how much light has been absorbed by the sample. By understanding this amount, we find out what concentration of the desired element our sample has.
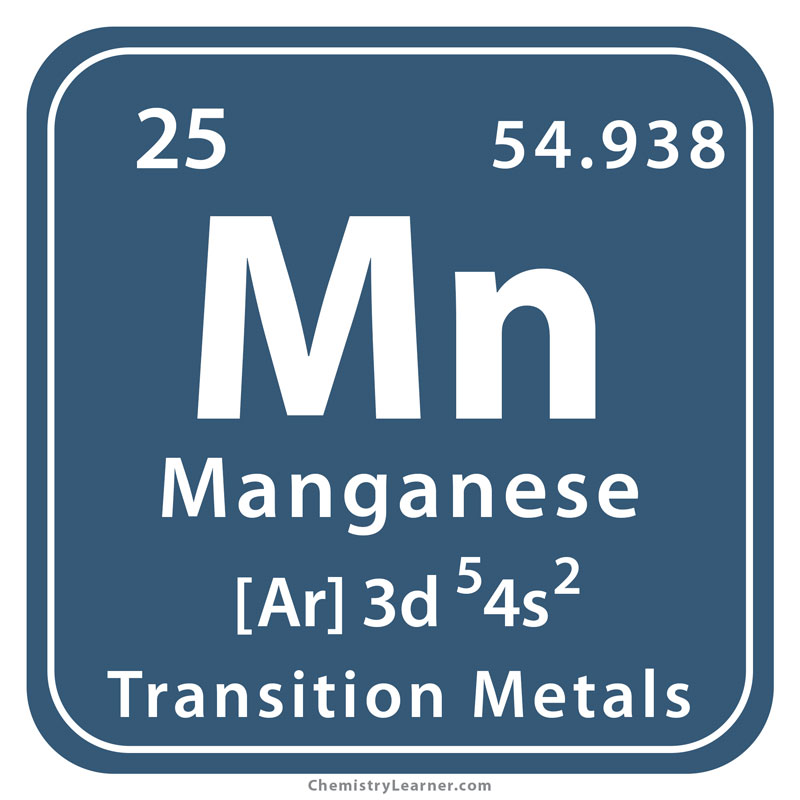
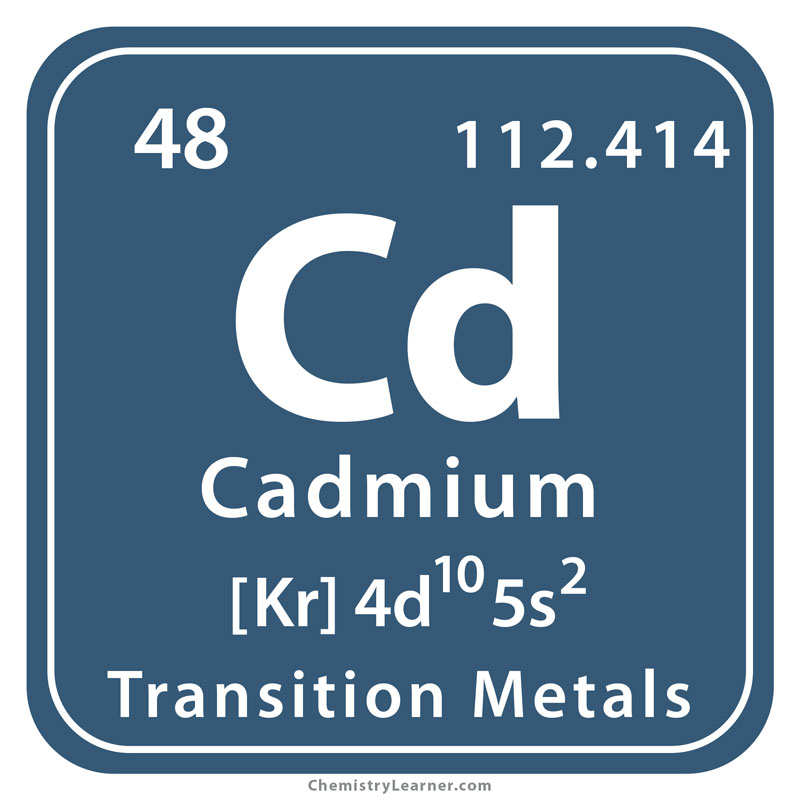
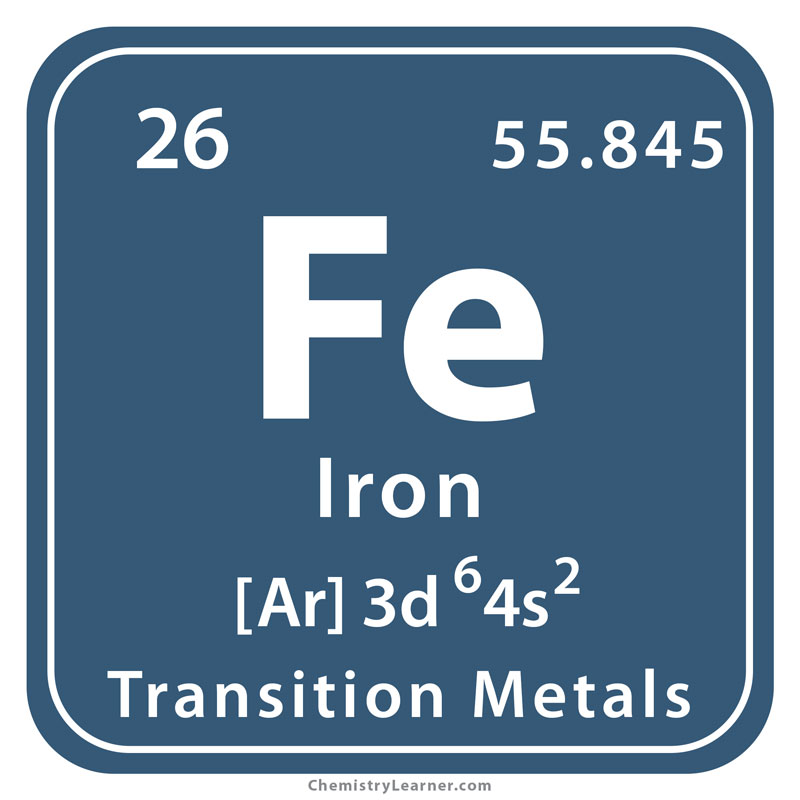
Faramin Asia Mineral Industrial Group is able to perform analysis tests with atomic absorption spectrometer for different elements. We see these elements below: